Electron Probability Distribution
Poster - Light
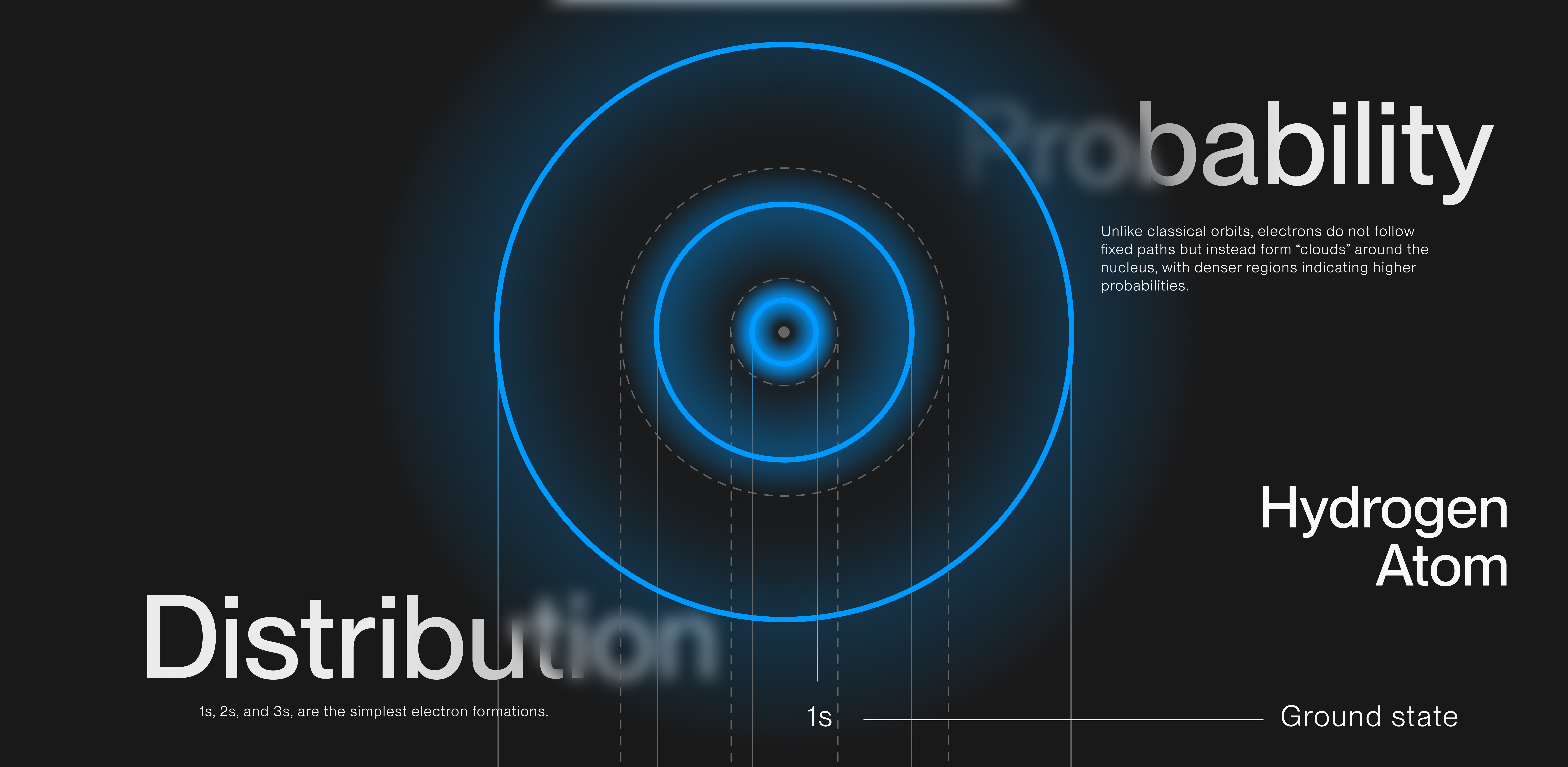
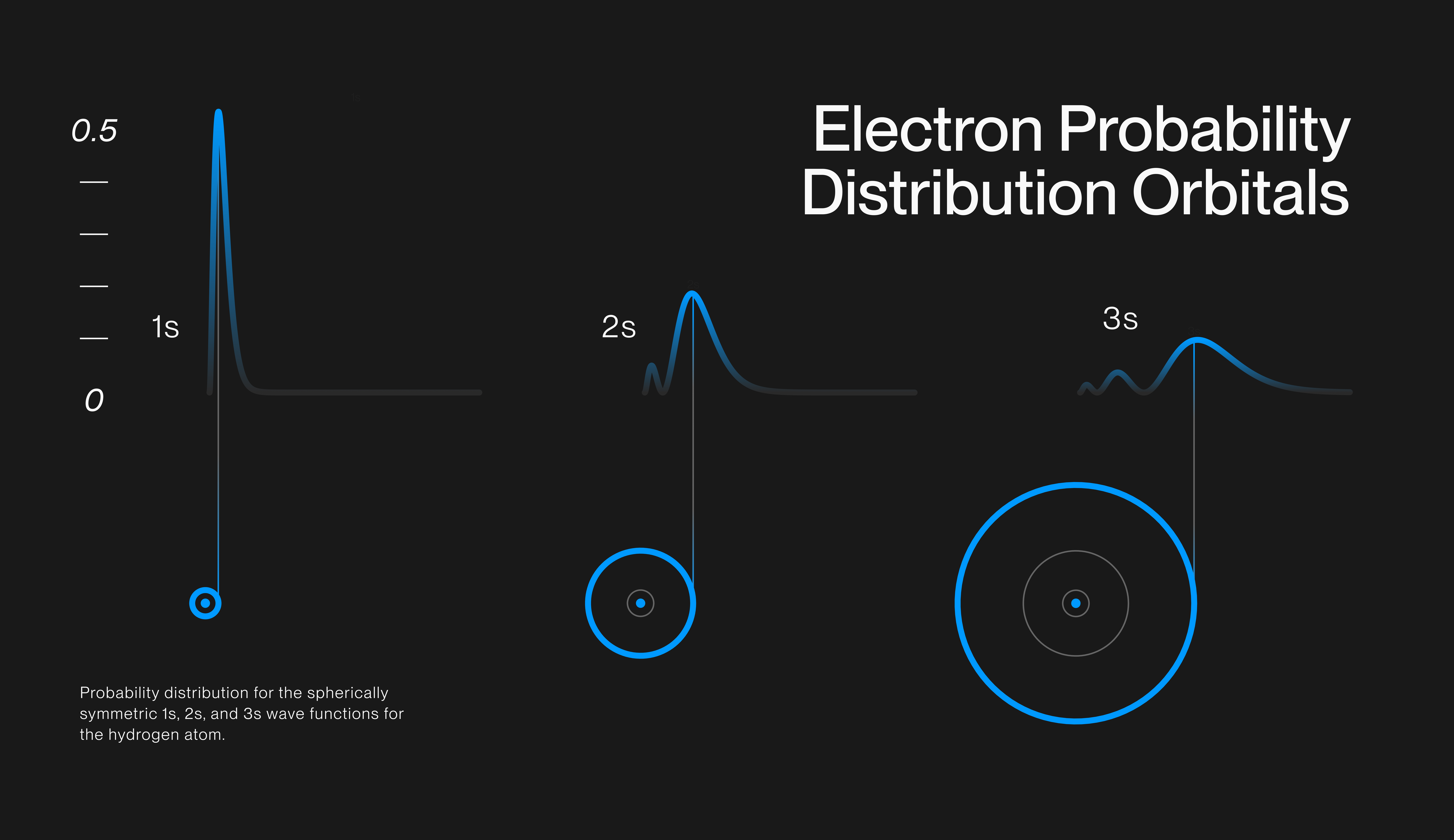
An electron's probability density describes the likelihood of finding an electron
in a particular region around the nucleus of an atom. Probability density is represented by 3D
clouds of probability, called orbitals, graphed in 2 dimensional space.
These graphs are solutions
to the Schrödinger equation, and the orbitals illustrate where an electron is most likely to be
found, reflecting the inherently probabilistic and quantized nature of quantum mechanics.