Hydrogen Emission Spectrum
Poster - Light
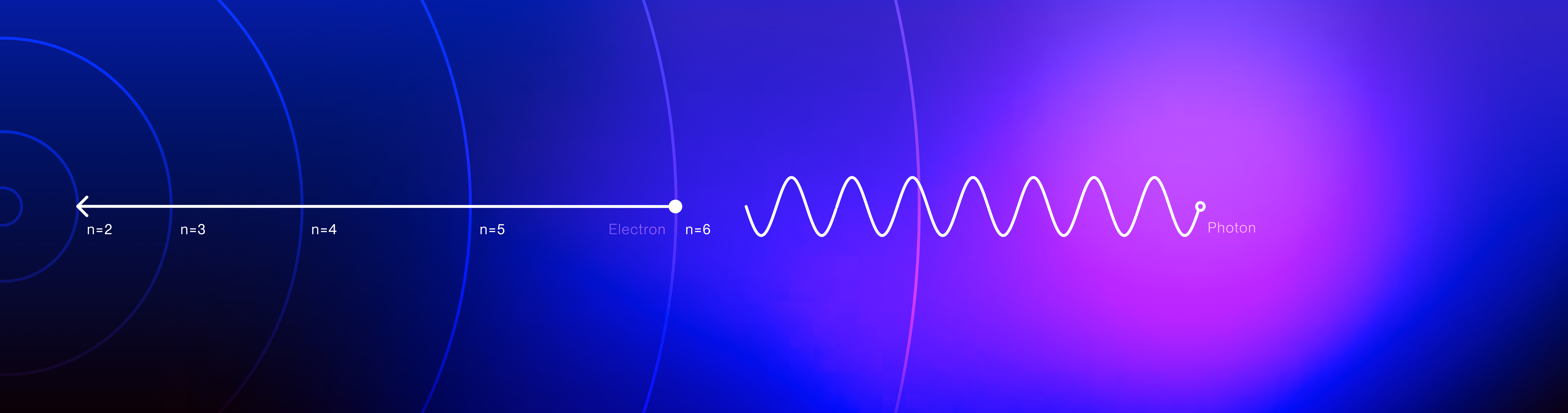
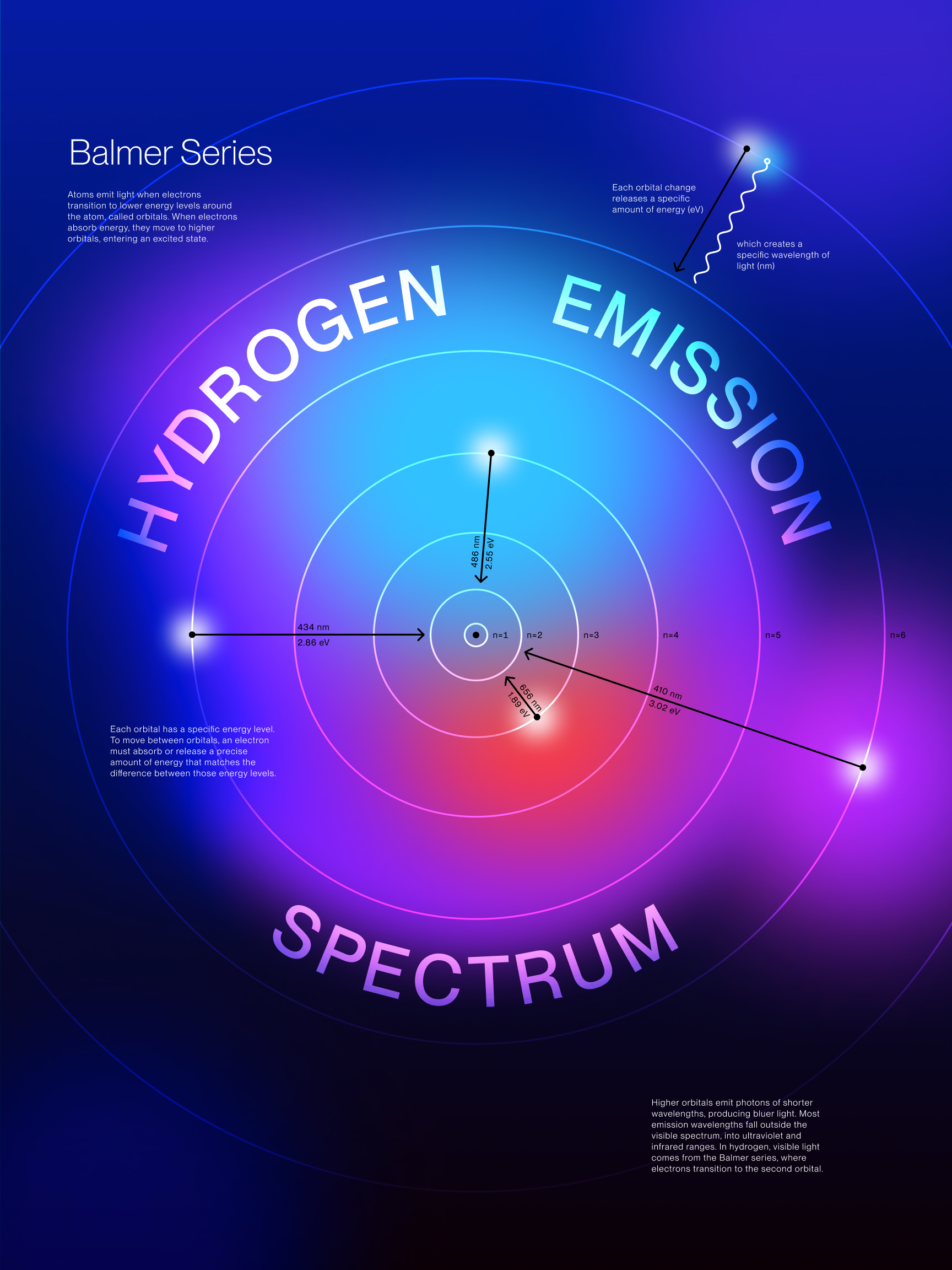
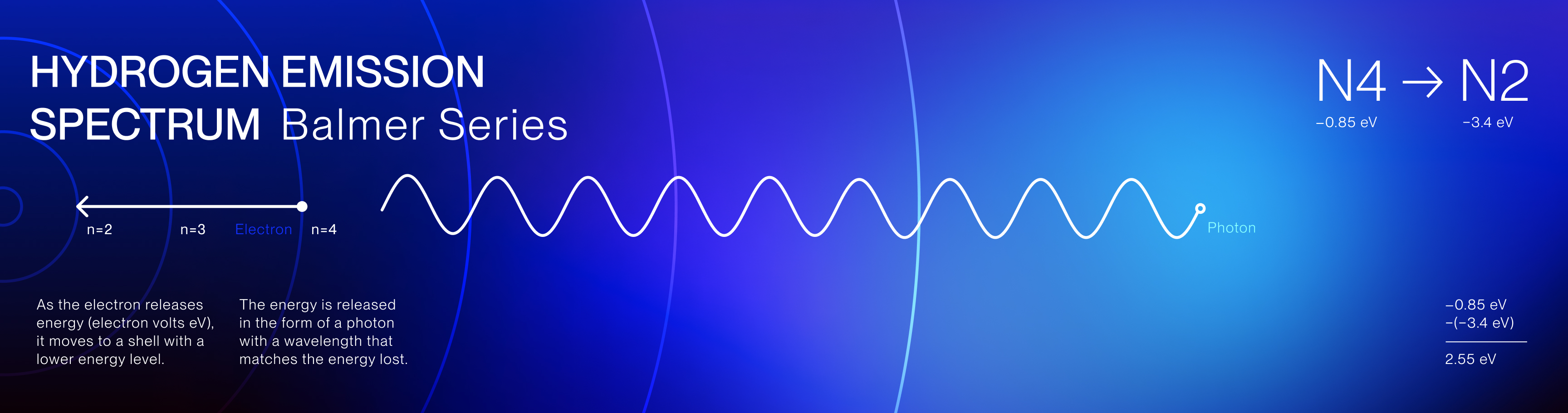
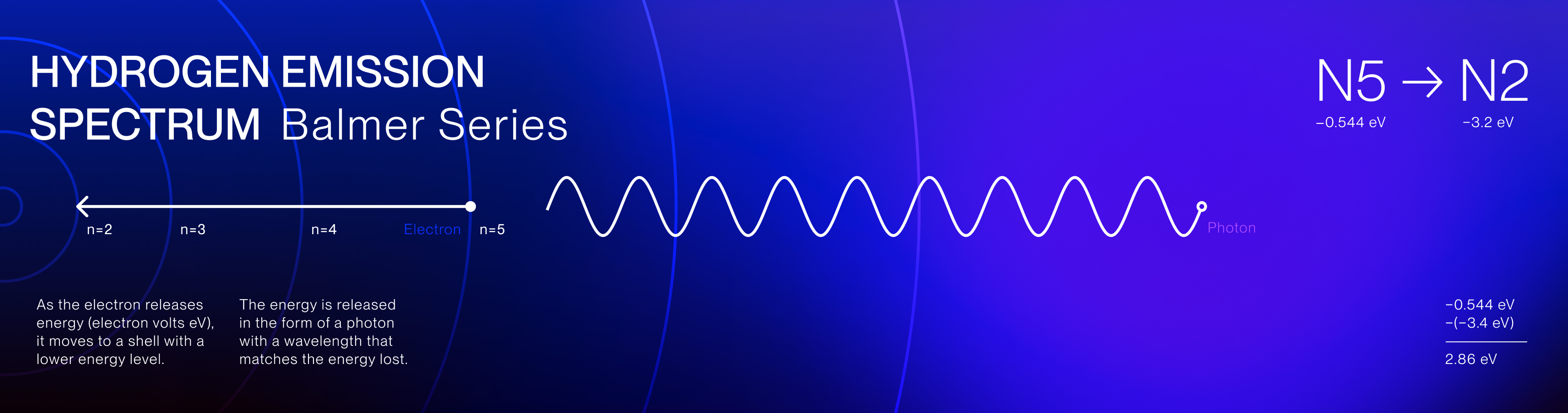
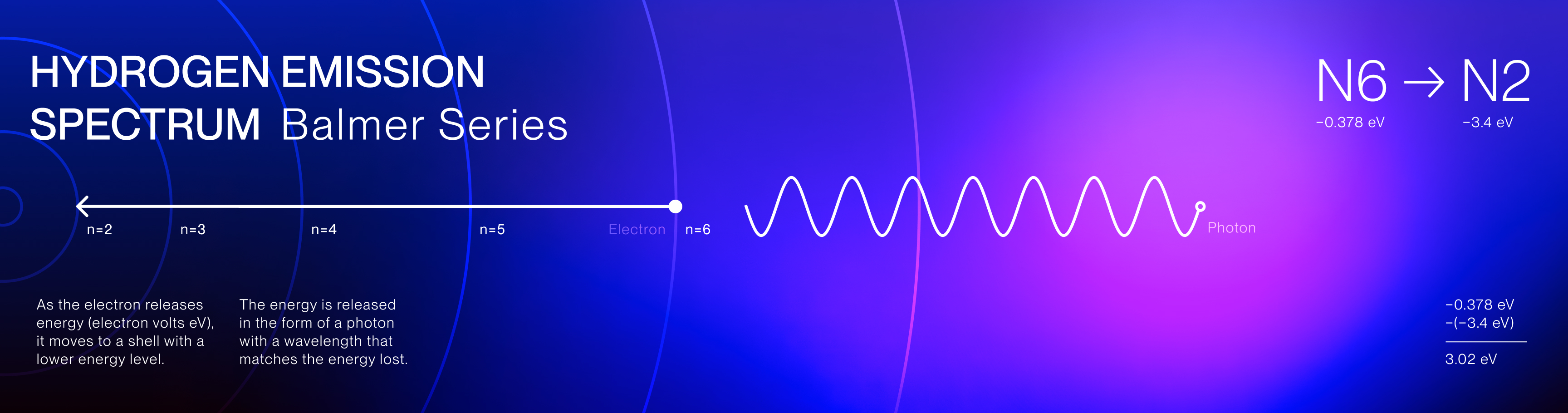
The Hydrogen Emission Spectrum is a series of spectral lines produced when hydrogen
gas absorbs and releases energy in the form of light. The spectrum consists of several series,
including the Balmer series, which is the only series of hydrogen's emissions in the visible light
spectrum.
These specific emission colors are unique to hydrogen and reveal the discrete energy transitions of
its electrons and the quantized nature of electron movement.